Voyager Medical Research
Everything we do is intended to reduce severe post-surgical complications and help save lives.
We do so by developing unique, proprietary polymers that are designed specifically to help prevent complications.
Our first product, ACE Surgical Suture, addresses the post-surgical complication of Incisional Hernia that is effecting up to 20% of patients undergoing a Laparotomy, resulting in over 5.3% mortality.
Our team is highly experienced and recognized in the field of wound-closure and biodegradable polymers.
The problem
In a time frame of 5 years, 1 of every 5 patients will develop a Post-Operative Ventral Hernia (POVH) after an abdominal surgery (Open or Laparoscopic).
POVH is the protrusion of internal organs through the incision of a previous surgery, it is a serious complication that is difficult to repair with even higher incidence of recurrence after a primary repair.
In addition many patients with POVH suffer acute pain, physical and psychological distress, high morbidity and high mortality (5.3%).
One of the main reasons for POVH has to do with the surgical sutures used for abdominal wall closure. Gold standard absorbable sutures such as PDS-II® (Ethicon) offer a maximum tensile strength of 8 weeks, meaning that after 8 weeks the tissue is supposed to be self-supporting. Unfortunately over 50% of patients have severe delays in wound-healing. Research shows that these patients require significantly more support time (over 16 weeks) for their abdominal wall before it could be considered self-supporting.
A surgical suture with extended tensile strength support will allow the tissue enough time to become self-sufficient and as a result reduce the incidence of POVH.
Voyager Surgical Suture
ACE Surgical Suture, is a monofilament, absorbable surgical suture, intended for abdominal wall closure.
The product is based on a co-polymer that was designed and validated in over 6 years of research at the Institute of Chemistry of the Hebrew University. ACE is composed of PPCA which is a unique co-polymer comprising Poly(Propylene Oxide) and Poly(Caprolactone) blocks.
Unlike competitor products that only provide 8 weeks of abdominal wall support, ACE offers as much as 5-6 months of support, enough to enable healing for all patients, low-risk and high risk alike.
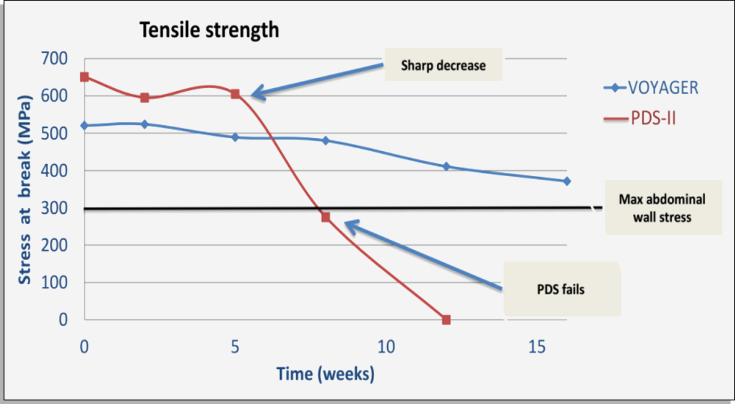
Our In-Vivo study show that when ACE is compared to the gold standard suture PDS-II, ACE is 74% stronger at 8 weeks and while PDS-II is no longer effective after 8 weeks ACE continues to show high and stable tensile strength at week 16 and over.
Up to 18 months post-implantation ACE fully degrades and is evacuated from the body safely.
In the picture: Violet suture (PDS II), Clear suture (ACE)
Perfect combination of Business-Science-Practice


